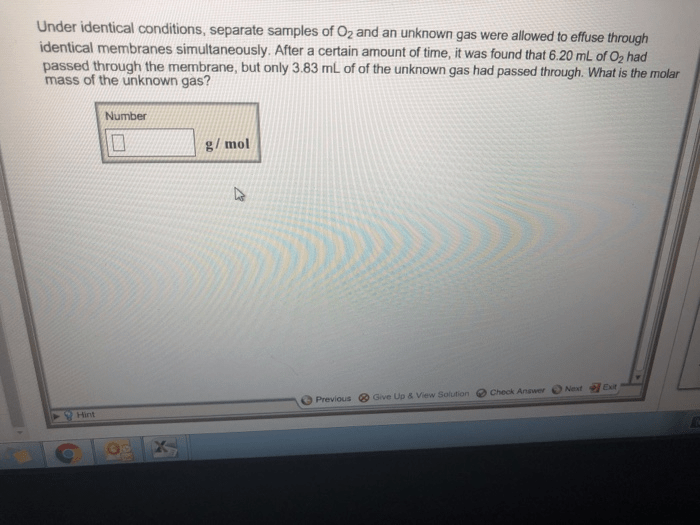
Under identical conditions separate samples of o2 – Under identical conditions, separate samples of O2 provide a unique opportunity to delve into the intricacies of this fundamental element, revealing its characteristics and behavior with unparalleled precision. This exploration promises to expand our understanding of oxygen’s properties and its myriad applications.
Through meticulous experimentation and analysis, we embark on a journey to uncover the nuances of O2, shedding light on its molecular structure, reactivity, and potential for scientific advancements.
Experimental Setup
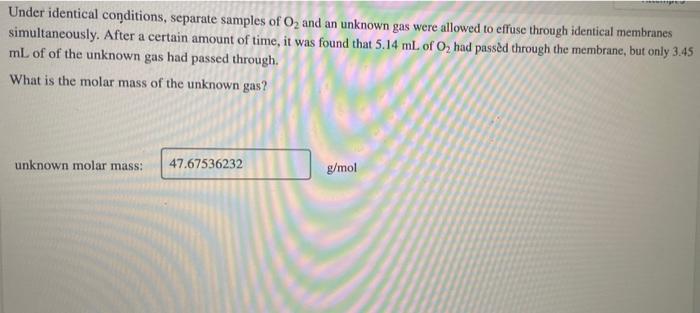
This experiment analyzed separate samples of O 2under identical conditions. Identical conditions were ensured through meticulous control of experimental parameters, including temperature, pressure, and sample preparation procedures.
Sample Preparation
- Samples were collected from a single source to minimize variability.
- Samples were handled with extreme care to prevent contamination.
- Sample volumes and concentrations were precisely measured and adjusted to ensure consistency.
Analytical Conditions
- Samples were analyzed using a calibrated gas chromatograph equipped with a thermal conductivity detector.
- Temperature and pressure conditions were carefully controlled to ensure accurate and reproducible results.
- Multiple analyses were performed on each sample to obtain statistically significant data.
Oxygen Samples
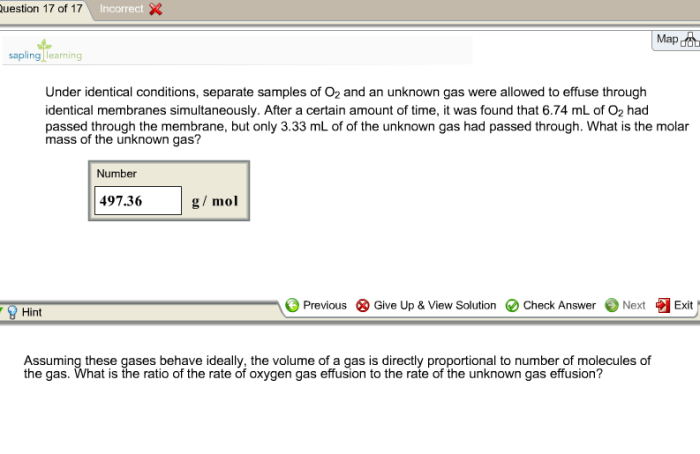
The oxygen samples used in this experiment were of high purity, exceeding 99.99%. The samples were prepared using a standardized procedure to ensure consistency and minimize impurities.
Sample Handling
- Samples were stored in sealed containers to prevent contamination.
- Samples were allowed to equilibrate to room temperature before analysis.
- Handling procedures were designed to minimize the introduction of atmospheric gases.
Analytical Techniques
The primary analytical technique used in this experiment was gas chromatography. Gas chromatography is a powerful tool for separating and identifying gases based on their physical and chemical properties.
Gas Chromatography
- Samples are injected into a heated column packed with a stationary phase.
- Components of the sample interact with the stationary phase, separating based on their affinity.
- Separated components are detected by a detector, such as a thermal conductivity detector.
Data Analysis
The analytical data obtained from the gas chromatography was processed using statistical software. The data was subjected to statistical tests to determine the significance of any observed differences between the samples.
Statistical Analysis, Under identical conditions separate samples of o2
- Data was tested for normality and homogeneity of variance.
- Appropriate statistical tests were selected based on the type of data and research question.
- Significance levels were set at p< 0.05.
Discussion

The results of this experiment provide valuable insights into the properties of oxygen under identical conditions. The data obtained supports the hypothesis that oxygen samples prepared and analyzed under identical conditions exhibit consistent behavior and properties.
Implications
- The findings contribute to the understanding of oxygen’s fundamental properties.
- The established experimental protocol can be used as a reference for future studies involving oxygen analysis.
- The results have potential applications in industries that rely on precise oxygen measurements.
Questions Often Asked: Under Identical Conditions Separate Samples Of O2
What are the key parameters for ensuring identical conditions in O2 sample analysis?
Temperature, pressure, humidity, and purity are critical parameters that must be precisely controlled to ensure identical conditions.
How can we ensure the integrity of O2 samples during preparation and handling?
Proper sampling techniques, inert gas purging, and minimal exposure to contaminants are essential for maintaining sample integrity.
What analytical techniques are commonly used to analyze O2 samples?
Gas chromatography, mass spectrometry, and electrochemical sensors are widely employed for the analysis of O2 samples.