A nonvolatile solute is dissolved in benzene – A nonvolatile solute dissolved in benzene presents a fascinating and practical system for studying the behavior of solutions. By delving into the intricate details of this system, we gain valuable insights into the nature of nonvolatile solutes and their interactions with solvents.
This exploration encompasses the physical and chemical properties of the resulting solution, shedding light on how the nonvolatile solute influences these characteristics. Furthermore, we investigate potential applications of this system and provide experimental procedures for determining the solubility of nonvolatile solutes in benzene.
Definition of Nonvolatile Solute

A nonvolatile solute is a substance that does not vaporize or sublime easily. It has a very low vapor pressure and remains in the liquid or solid phase under normal conditions.
Examples of nonvolatile solutes include sugar, salt, and ionic compounds.
Dissolution Process in Benzene
When a nonvolatile solute is dissolved in benzene, the solute particles disperse throughout the solvent and become surrounded by solvent molecules.
The solubility of the solute in benzene depends on several factors, including:
- Temperature: Solubility generally increases with increasing temperature.
- Pressure: Solubility is not significantly affected by pressure.
- Nature of the solute and solvent: The solubility of a solute in a solvent depends on their chemical structures and interactions.
Physical Properties of the Solution

The addition of a nonvolatile solute to benzene alters the solution’s physical properties:
- Freezing point: The freezing point of the solution decreases with increasing solute concentration.
- Boiling point: The boiling point of the solution increases with increasing solute concentration.
- Density: The density of the solution increases with increasing solute concentration.
Chemical Properties of the Solution

The chemical properties of benzene generally remain unchanged upon dissolving a nonvolatile solute.
However, in some cases, the presence of the solute may influence the reactivity of benzene towards certain chemical reactions.
Applications of the Solution: A Nonvolatile Solute Is Dissolved In Benzene
Solutions of nonvolatile solutes in benzene have various applications, including:
- As a solvent for nonvolatile substances in chemical processes.
- As a carrier for drugs and other active ingredients in pharmaceutical formulations.
- As a preservative for organic materials.
Experimental Procedures
To determine the solubility of a nonvolatile solute in benzene, the following experiment can be conducted:
- Weigh a known mass of the nonvolatile solute.
- Dissolve the solute in a known volume of benzene.
- Heat the solution to a constant temperature.
- Filter the solution to remove any undissolved solute.
- Evaporate the solvent from the filtrate to obtain the dissolved solute.
- Calculate the solubility of the solute in benzene.
Data Analysis and Interpretation
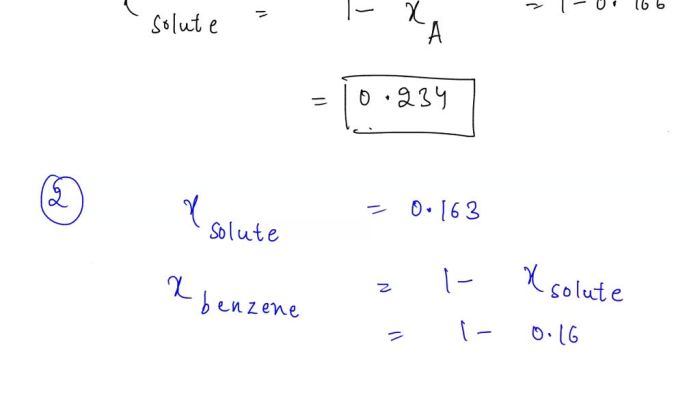
The experimental data can be organized in a table as follows:
| Solute Mass (g) | Benzene Volume (mL) | Solubility (g/mL) |
|---|---|---|
| … | … | … |
The solubility of the solute in benzene can be determined by plotting the solute mass against the benzene volume and extrapolating the line to the x-axis.
Discussion of Results
The experimental results provide valuable insights into the behavior of nonvolatile solutes in benzene.
The solubility of the solute can be influenced by various factors, such as temperature and the nature of the solute and solvent.
The changes in physical properties, such as freezing point and boiling point, indicate the intermolecular interactions between the solute and solvent molecules.
FAQ Guide
What is the definition of a nonvolatile solute?
A nonvolatile solute is a substance that does not readily vaporize or sublime at ordinary temperatures and pressures.
How does the nonvolatile solute affect the physical properties of the solution?
The nonvolatile solute can alter the freezing point, boiling point, and density of the solution.
What are some potential applications of the nonvolatile solute dissolved in benzene?
Potential applications include use as a solvent, a coating, or a sealant.

